IMPORTANT SAFETY INFORMATION
INDICATIONS
EDARBI is an angiotensin II receptor blocker (ARB) indicated for the treatment of hypertension in adults, to lower blood pressure and EDARBYCLOR is an angiotensin II receptor blocker (ARB) and a thiazide-like diuretic combination product indicated for the treatment of hypertension to lower blood pressure. EDARBI may be used either alone or in combination with other antihypertensive agents. EDARBYCLOR may be used if a patient is not adequately controlled on monotherapy or as initial therapy if multiple drugs are needed to help achieve blood pressure goals. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. There are no controlled trials demonstrating risk reduction with EDARBI or EDARBYCLOR, but trials with chlorthalidone and at least one antihypertensive agent pharmacologically similar to azilsartan medoxomil have demonstrated such benefits. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on hypertension goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). EDARBI and EDARBYCLOR may be used with other antihypertensive agents.
WARNING: FETAL TOXICITY
See full Prescribing Information for complete boxed warnings.
- When pregnancy is detected, discontinue EDARBI or EDARBYCLOR as soon as possible.
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
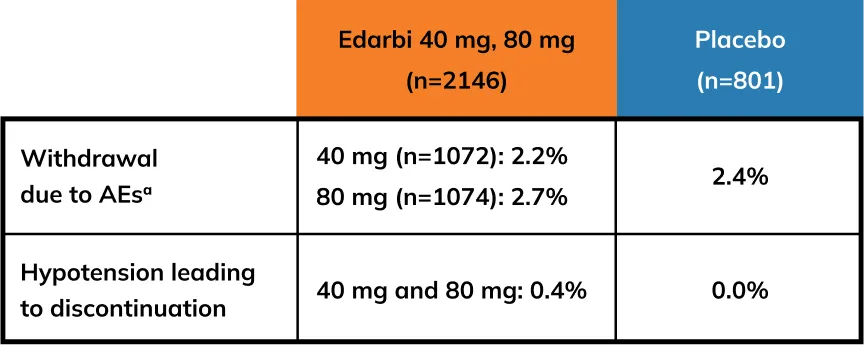
WARNINGS AND PRECAUTIONS
EDARBYCLOR is contraindicated in patients with anuria.
Do not coadminister aliskiren-containing products with EDARBI or EDARBYCLOR in patients with diabetes.
Fetal Toxicity: EDARBI or EDARBYCLOR can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neoatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue EDARBI or EDARBYCLOR as soon as possible. Thiazides cross the placental barrier and appear in cord blood and may be associated with adverse reactions, including fetal or neonatal jaundice and thrombocytopenia.
Hypotension in Volume - or Salt-Depleted Patients: In patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients (e.g., those being treated with high doses of diuretics), symptomatic hypotension may occur after initiation of treatment with EDARBI or EDARBYCLOR. Such patients are probably not good candidates to start therapy with more than one drug; therefore, correct volume prior to administration of EDARBYCLOR. Correct volume or salt depletion prior to administering EDARBI, or start treatment at 40 mg. If hypotension does occur, the patient should be placed in the supine position and, if necessary, given an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
Impaired Renal Function: Monitor for worsening renal function in patients with renal impairment. Consider withholding or discontinuing EDARBYCLOR if progressive renal impairment becomes evident. As a consequence of inhibiting the renin-angiotensin system, changes in renal function may be anticipated in susceptible individuals treated with EDARBI or EDARBYCLOR. In patients whose renal function may depend on the activity of the renin-angiotensin system (e.g., patients with severe congestive heart failure, renal artery stenosis, or volume depletion), treatment with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers has been associated with oliguria or progressive azotemia and rarely with acute renal failure and death. Similar results may be anticipated in patients treated with EDARBI or EDARBYCLOR. In studies of ACE inhibitors in patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or blood urea nitrogen have been reported. There has been no long-term use of azilsartan medoxomil in patients with unilateral or bilateral renal artery stenosis, but similar results are expected. In patients with renal disease, chlorthalidone may precipitate azotemia. If progressive renal impairment becomes evident, as indicated by increased blood urea nitrogen, consider withholding or discontinuing diuretic therapy.
Serum Electrolyte Imbalances: Thiazide diuretics can cause hyponatremia and hypokalemia. Drugs that inhibit the renin angiotensin system can cause hyperkalemia. Hypokalemia is a dose-dependent adverse reaction that may develop with chlorthalidone. Co-administration of digitalis may exacerbate the adverse effects of hypokalemia. Monitor serum electrolytes periodically. EDARBYCLOR attenuates chlorthalidone-associated hypokalemia. In patients with normal potassium levels at baseline, 1.7% of EDARBYCLOR-treated patients, 0.9% of azilsartan medoxomil-treated patients, and 13.4% of chlorthalidone-treated patients shifted to low potassium values (less than 3.4 mmol/L).
Hyperuricemia: Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving chlorthalidone or other thiazide diuretics.
ADVERSE REACTIONS (AEs):
- The most common AE that occurred more frequently with EDARBI than placebo in adults was diarrhea (2% vs 0.5%).
- AEs occurred at an incidence of ≥2% of EDARBYCLOR-treated patients and greater than azilsartan medoxomil or chlorthalidone were dizziness (8.9%) and fatigue (2.0%).
The incidence of consecutive increases of creatinine (≥50% from baseline and >ULN) was 2.0% in patients treated with the recommended doses of Edarbyclor compared with 0.4% and 0.3% with azilsartan medoxomil and chlorthalidone, respectively.
DRUG INTERACTIONS:
- Monitor renal function periodically in patients receiving EDARBI or EDARBYCLOR and NSAIDs who are also elderly, volume-depleted (including those on diuretics), or who have compromised renal function, as deterioration of renal function, including possible acute renal failure, may result. These effects are usually reversible. NSAIDs may reduce the antihypertensive effect of EDARBI or EDARBYCLOR.
- Dual blockade of the renin-angiotensin system (RAS) with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on EDARBI or EDARBYCLOR and other agents that affect the RAS. Do not coadminister aliskiren with EDARBI or EDARBYCLOR in patients with diabetes or renal impairment (GFR <60 mL/min).
- Renal clearance of lithium is reduced by diuretics, such as chlorthalidone, increasing the risk of lithium toxicity. Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II receptor agonists. Monitor serum lithium levels during concomitant use.
The Important Safety Information does not include all the information needed to use EDARBI and EDARBYCLOR safely and effectively.
For further information, please see complete Prescribing Information for EDARBI and EDARBYCLOR.
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449 or to the FDA at www.fda.gov/medwatch or call 1-800-FDA-1088.